Introduction
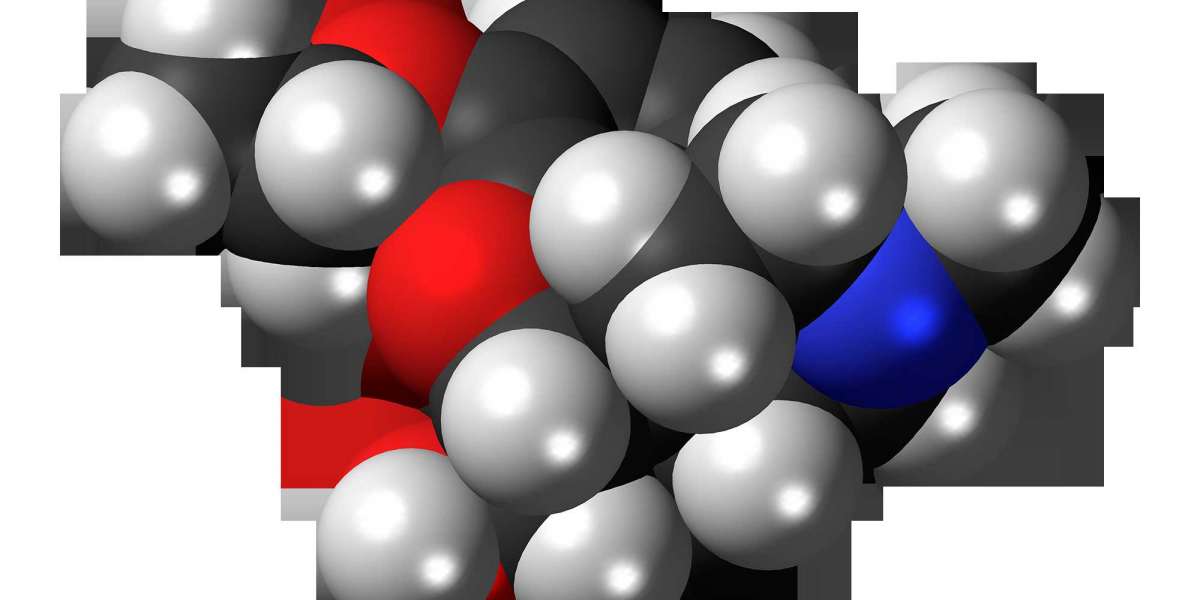
Ethylmorphine, also known as Dionine or codethyline, is an opioid analgesic and antitussive (cough suppressant) that belongs to the morphinane alkaloid class. With the chemical formula C19H23NO3, it is a semi-synthetic derivative of morphine, structurally similar to codeine. Ethylmorphine exerts its effects by binding to opioid receptors in the central nervous system, primarily the mu-opioid receptors, which leads to a reduction in pain perception and suppression of the cough reflex. It is often metabolized in the liver to morphine, contributing to its analgesic properties. While not widely marketed in the United States due to its classification as a controlled substance with potential for abuse, ethylmorphine is approved and utilized in various countries globally for the short-term treatment of moderate pain and for managing dry, irritating coughs. Its therapeutic value lies in its dual action as both a pain reliever and a cough suppressant.
The ethylmorphine industry is influenced by a complex interplay of medical demand, regulatory controls, and the broader landscape of pain and cough management. Its primary market drivers stem from the persistent need for effective opioid analgesics for acute or short-term pain relief and its utility as a cough suppressant, particularly for dry, non-productive coughs. In regions where it is medically approved and available, the increasing burden of respiratory illnesses and conditions requiring pain management contributes to its sustained demand. The cost-effectiveness of older, established opioid derivatives compared to newer, more expensive alternatives can also play a role in its market presence in certain healthcare systems. Looking ahead, several key trends are shaping the industry. The stringent global regulations on opioid substances due to concerns about abuse and addiction significantly impact the production, distribution, and prescription of ethylmorphine, leading to controlled and often restricted access. This regulatory environment encourages a shift towards non-opioid alternatives for pain and cough, which may limit market growth. However, ongoing pharmaceutical research exploring novel formulations or combinations that enhance its therapeutic benefits while minimizing side effects or abuse potential could provide new avenues. The availability and price of raw materials from the opium poppy also influence the supply chain of semi-synthetic opioids like ethylmorphine. Furthermore, public health campaigns aimed at reducing opioid dependency could lead to further restrictions, while in regions with less developed healthcare infrastructure, its accessibility as an affordable option might be sustained.
Project Scope and Overview
IMARC’s new report titled “Ethylmorphine Manufacturing Plant Project Report 2025: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue,” provides a comprehensive roadmap for setting up an ethylmorphine manufacturing plant. The study encompasses all the essential information needed to enter the ethylmorphine industry. This report offers an in-depth evaluation of the ethylmorphine manufacturing plant cost, including detailed insights into ethylmorphine manufacturing plant machinery cost, enabling readers to understand recurring operational expenditures and return on investment. It also presents a practical ethylmorphine manufacturing business plan, serving as a valuable resource for entrepreneurs, investors, researchers, consultants, business strategists, and anyone with an interest or stake in the ethylmorphine sector. Moreover, it outlines the ethylmorphine manufacturing plant setup cost, guiding users through the capital planning, machinery selection, and resource allocation stages essential for launching production successfully.
Manufacturing Process and Technical Workflow
This report offers detailed information related to the process flow and the unit operations involved in an ethylmorphine manufacturing plant project. Moreover, information related to raw material requirements and mass balance has further been provided in the report with a list of necessary technical tests as well as quality assurance criteria.
Aspects Covered
- Product Overview
- Unit Operations Involved
- Mass Balance and Raw Material Requirements
- Quality Assurance Criteria
- Technical Tests
Request for a Sample Report: https://www.imarcgroup.com/ethylmorphine-manufacturing-plant-project-report/requestsample
Infrastructure and Setup Requirements
This section presents a comprehensive analysis of key considerations involved in establishing an ethylmorphine manufacturing plant. It covers critical aspects such as land location, selection criteria, strategic significance of the site, environmental impact, and associated land acquisition costs. In addition, the report outlines the proposed plant layout along with the primary factors influencing its design. Furthermore, it provides detailed insights into various operational requirements and expenditures, including those related to packaging, utilities, machinery, transportation, raw materials, and human resources.
- Land, Location and Site Development
- Plant Layout
- Machinery Requirements and Costs
- Raw Material Requirements and Costs
- Packaging Requirements and Costs
- Transportation Requirements and Costs
- Utility Requirements and Costs
- Human Resource Requirements and Costs
Browse the Full Report with the Table of Contents: https://www.imarcgroup.com/ethylmorphine-manufacturing-plant-project-report
Financial Projections and Economic Viability
This section provides a comprehensive economic analysis for establishing a ethylmorphine manufacturing plant. It encompasses a detailed evaluation of capital expenditure (CapEx), operating expenditure (OpEx), taxation, and depreciation. Additionally, the report includes profitability analysis, payback period estimation, net present value (NPV), projected income statements, liquidity assessment, and in-depth examinations of financial uncertainty and sensitivity parameters.
- Capital Investments
- Operating Costs
- Expenditure Projections
- Revenue Projections
- Taxation and Depreciation
- Profit Projections
- Financial Analysis
Key Considerations for Plant Design and Operations:
Production Capacity:
The selection of machinery and the design of the plant layout should be aligned with the intended scale of production, which may vary from small-scale operations to large industrial facilities. This alignment ensures optimal utilization of space, resources, and production capabilities.
Automation Levels:
The degree of automation should be adjusted based on factors such as labor availability, budget constraints, and the level of technical expertise. Options may range from semi-automated systems to fully automated solutions, allowing for flexibility in capital investment and operational efficiency.
Location Adaptation:
Plant location should be strategically selected to align with local market demand, ensure proximity to raw material sources, leverage available labor, and comply with regional regulatory requirements. These factors collectively contribute to improved operational efficiency and cost optimization.
Product Flexibility:
The plant should be equipped with processes and machinery capable of accommodating a variety of product specifications. This flexibility enables manufacturers to respond to diverse and evolving market demands effectively.
Sustainability Features:
Incorporating sustainable practices is essential. This includes the integration of renewable energy sources, implementation of efficient waste management systems, and use of energy-efficient machinery to meet environmental standards and long-term sustainability objectives.
Raw Material Sourcing:
The supply chain strategy should be customized to ensure reliable and cost-effective sourcing of raw materials. This approach should consider client-specific requirements and regional supply dynamics to maintain consistent production and manage input costs.
About Us: IMARC Group is a leading global market research and management consulting firm. We specialize in helping organizations identify opportunities, mitigate risks, and create impactful business strategies.
Our expertise includes:
- Market Entry and Expansion Strategy
- Feasibility Studies and Business Planning
- Company Incorporation and Factory Setup Support
- Regulatory and Licensing Navigation
- Competitive Analysis and Benchmarking
- Procurement and Supply Chain Research
- Branding, Marketing, and Sales Strategy
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145